Abstract
Background: Anticoagulation (AC) is the cornerstone of treatment for venous thromboembolism (VTE). Direct oral anticoagulants (DOACs) have become the standard of care during the acute and extended phases of therapy for VTE, mostly due to their ease of use, efficacy, and safety profiles. There is a 9.2% prevalence of severe obesity (BMI>40) in Americans as of 2017-2020 with a 6.2-fold increased risk of VTE in obese individuals. The 2021 International Society on Thrombosis and Haemostasis (ISTH) guidelines recommend using standard dosages of DOACs in patients with a BMI >40 kg/m2 or weight >120 kg. Despite these recommendations, their use in severely obese individuals at extremes of weight like our patient with a BMI > 70 kg/m2 remains uncertain.
Case Presentation
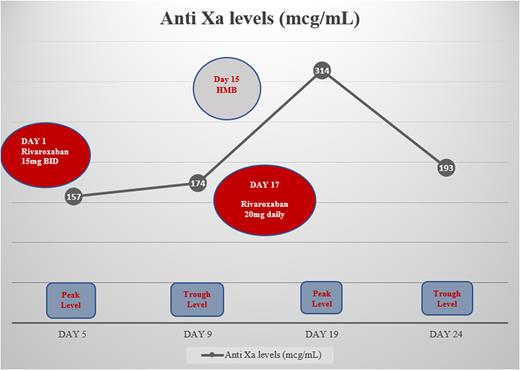
Herein, we present a 38-year-old morbidly obese female with no significant past medical history who presented with an acute worsening of dyspnea on exertion saturating 60% on room air. The diagnosis of intermediate-high risk pulmonary embolism (PE) was made based on computed tomographic angiography (CTA) with right ventricular strain and elevated cardiac biomarkers. A concomitant proximal DVT from the left common femoral to the distal peroneal vein was also noted. The patient's weight was 243kg with a calculated BMI of 72.3 kg/m2. After the patient had received parenteral anticoagulation with heparin for 4 days, it was decided to switch to Rivaroxaban 15mg BID for 21 days followed by 20 mg daily. With a history of irregular menses, the patient developed heavy menstrual bleeding (HMB) following the initiation of rivaroxaban. Her hemoglobin remained stable with no blood transfusion requirements. However, this led to the decision to switch to rivaroxaban 20 mg PO daily earlier than planned due to a lack of improvement in menstrual bleeding which was considered to be clinically relevant non-major bleeding (CRNMB). Despite this switch, there was no effect on efficacy and the patient did not develop any signs or symptoms of VTE recurrence during her prolonged hospital stay with a significant improvement in her oxygenation levels and VTE symptomatology.
In our patient, we demonstrated the use of rivaroxaban in a morbidly obese individual with a BMI>70 by achieving therapeutic anti-Xa levels. Figure 1 shows the therapeutic peak and trough anti-Xa levels throughout the patient's hospitalization. The highest Anti Xa level of 314 mcg/mL was reported on day 19 correlating with the patient's menorrhagia, which self-limited with the transition from rivaroxaban 15 mg twice daily to 20 mg daily.
Discussion: Two pivotal clinical trials, the EINSTEIN-DVT/PE trials, where 14% of patients in both arms weighed >100 kg, showed that patients on rivaroxaban (15 mg BID for 3 weeks, followed by 20 mg once daily) vs those on enoxaparin followed by a vitamin K antagonist, had no significant difference in recurrence rates of VTE and bleeding rates [Moore KT, et Al. Influences of Obesity, Am J Med 2017]. A recent post-hoc analysis of the EINSTEIN DVT/PE trials also portrayed no correlation between weight/BMI and risk of recurrent VTE or bleeding in those who received rivaroxaban. Our case illustrates the attainment of therapeutic anti-Xa levels with no recurrent VTE in our morbidly obese patient. Despite her heavy menstrual bleeding, the bleeding was controlled once the switch to rivaroxaban 20mg daily was completed. Another study in which its heaviest participant at 172 kg, showed compelling evidence that morbid obesity has minimal influence on the safety and efficacy of rivaroxaban [Speed V, et al. Fixed dose rivaroxaban. JTH 2020]. This remains true for our patient at 243 kg.
Conclusion: Our case demonstrates that standard doses of rivaroxaban for the treatment of DVT/PE in individuals with morbid obesity with BMI>70 kg/m2 can be effective, and safer for the initial phase of treatment for VTE and in prevention of VTE recurrence. It also portrays that increased dosages attempting to account for high bodyweight/fat can potentially result in major bleeding events. Despite the 2021 ISTH guidelines, further prospective studies and randomized controlled trials are warranted, to assess the safety and efficacy of DOACs in VTE, particularly in such challenging clinical scenarios like morbid obesity. Alongside clinical assessment, additional studies will be essential to evaluate the need for universal and external validation of anti-Xa levels in patients taking DOACs.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal